Published
Last 1 week
Last 30 days
Latest 24 hours
Published
Profession
Industry
Seniority
13
jobs
MRC/UVRI Uganda Research Unit on AIDS
Entebbe, Uganda
MRC/UVRI Uganda Research Unit on AIDS
Entebbe, Uganda
MRC/UVRI Uganda Research Unit on AIDS
Entebbe, Uganda
MRC/UVRI Uganda Research Unit on AIDS
Entebbe, Uganda
National Agricultural Research Organization (NARO)
Entebbe, Uganda
EMERGENCY Life Support for Civilian War Victims (EMERGENCY)
Entebbe, Uganda
EMERGENCY Life Support for Civilian War Victims (EMERGENCY)
Entebbe, Uganda
EMERGENCY Life Support for Civilian War Victims (EMERGENCY)
Entebbe, Uganda
EMERGENCY Life Support for Civilian War Victims (EMERGENCY)
Entebbe, Uganda
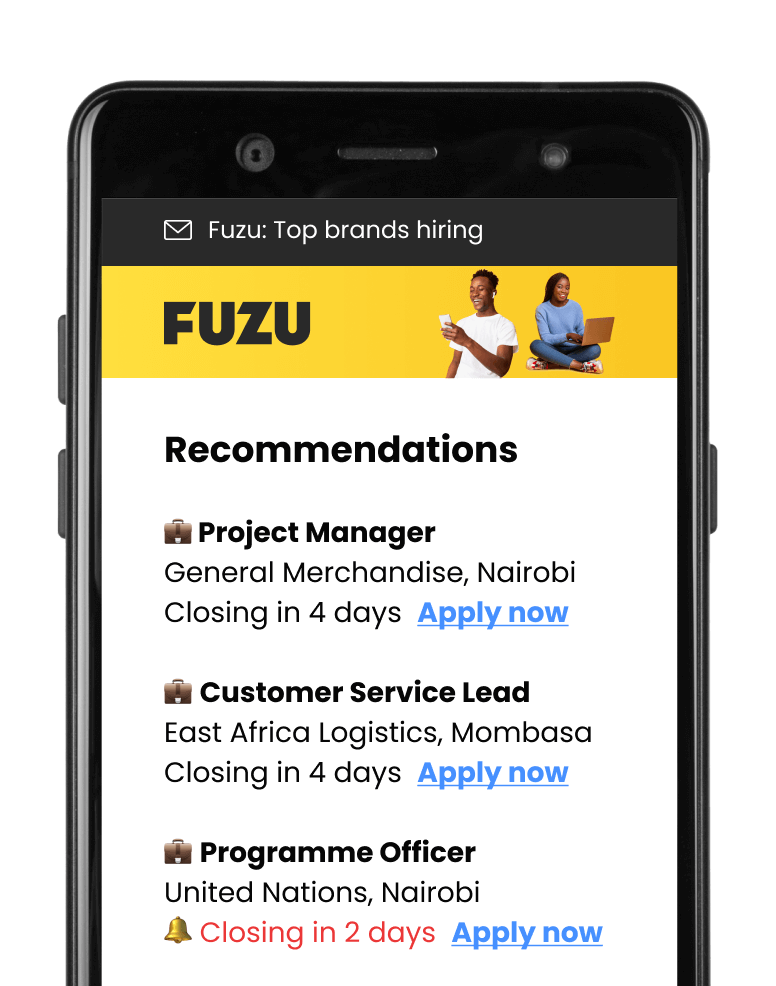
Get personalised job alerts directly to your inbox!
EMERGENCY Life Support for Civilian War Victims (EMERGENCY)
Entebbe, Uganda
Closing: Apr 19, 2024
1 day remainingPublished: Apr 15, 2024 (3 days ago)
Education:
Work experience:
Language skills:
Contract Type:
Sign up to view job details.
The post holder will participate in conducting immunology laboratory analyses under the supervision of the line manager.
Requirements
• Minimum of a master’s degree in a biological sciences field from a recognised Ugandan university;
• Good Clinical Practice and Clinical Laboratory Practice certification;
• Minimum two years of experience working in a laboratory technologist (or related) role in a busy research setting;
• Experience in processing blood samples from research study participants;
• Experience in conducting immunoassays (such as ELISA, ELISpot, Luminex, Flow cytometry);
• Ability to learn and conduct assays required, according to study protocols;
• Should possess Analytical and conceptual thinking skills;
• Thoroughness;
• Reliability; and, Concern for excellence.
The post holder will participate in conducting immunology laboratory analyses under the supervision of the line manager.
Requirements
• Minimum of a master’s degree in a biological sciences field from a recognised Ugandan university;
• Good Clinical Practice and Clinical Laboratory Practice certification;
• Minimum two years of experience working in a laboratory technologist (or related) role in a busy research setting;
• Experience in processing blood samples from research study participants;
• Experience in conducting immunoassays (such as ELISA, ELISpot, Luminex, Flow cytometry);
• Ability to learn and conduct assays required, according to study protocols;
• Should possess Analytical and conceptual thinking skills;
• Thoroughness;
• Reliability; and, Concern for excellence.
• Receiving, processing, and storing of study samples according to SOPs and study protocols;
• Planning and performing SOP-guided laboratory techniques including;
Enzyme-linked Immunosorbent assays (ELISAs)
Enzyme-linked Immunosorbent spot (ELISpot) assays
Peripheral blood mononuclear cell (PBMC) isolation from whole blood, and subsequent storage
Whole blood and PBMC stimulation assays
Multiplex bead array assays such as Luminex®
Flow cytometry
DNA extraction and polymerase chain reaction (PCR)
• Analysis of laboratory results, with the support of the line manager, and preparing reports on experiments;
• Ensuring that required reagents and supplies are in sufficient stocks and are stored appropriately;
• Finding and reading literature relevant to the work;
• Participate in writing publications, sometimes as lead author;
• Participating in sharing knowledge through laboratory meetings and journal clubs;
• Preparing and reviewing standard operating procedures;
• Implementing GCLP practices and engaging in proactive problem prevention of GCLP practice;
• Entering and cleaning laboratory data; and,
• Helping with other laboratory procedures and projects as and when required by the line manager
Line Management Responsibilities;
• Provide mentorship to one or more laboratory technicians; and,
• Supervise students working in the laboratory.

Applications submitted via Fuzu have 32% higher chance of getting shortlisted.